Zolate Prescribing Information
Package insert / product label
Generic name: folic acid, cholecalciferol
Dosage form: capsule
Drug class: Vitamins
Medically reviewed by Drugs.com. Last updated on Mar 25, 2024.
The Zolate brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
On This Page
Zolate Description
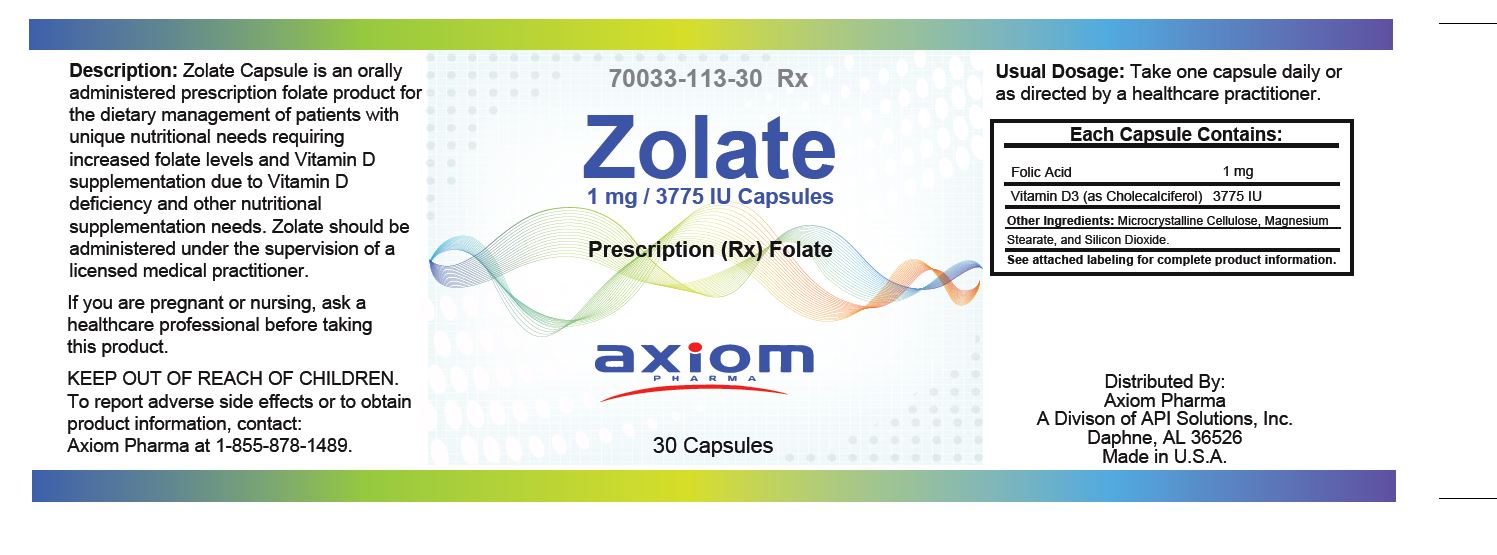
Zolate is an orally-administered (capsule) prescription folate product specifically formulated for the dietary management of patients with unique nutritional needs due to medical conditions and disease states requiring increased folate levels and specific Vitamin D supplementation levels.
Contraindications
This product is contraindicated in patients with a known hypersensitivity to any of the components contained in this product. This product is contraindicated for individuals with conditions for which any of the ingredients are contraindicated.
Warnings and Precautions
Caution is recommended in patients with a history of bipolar illness, as mood elevation is possible in this population.
Patients taking anticonvulsant medications should also exercise caution before taking this product, as folate may (i) interfere with anticonvulsant medication, and/or (ii) lower seizure threshold. Conversely, anticonvulsant medications may interfere with folate metabolism, although the exact mechanism of action is not clear or well understood.
Patients undergoing cancer treatment should consult their licensed medical practitioner for advice.
Folate alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folate in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission may occur while neurological manifestations progress.
Adverse Reactions/Side Effects
Allergic reactions have been reported following the use of oral and parenteral folate. Mild transient diarrhea, polycythemia vera, itching, transitory exanthema and the feeling of swelling of the entire body has been associated with cobalamin. Nausea, vomiting, diarrhea, transient skin rash, flushing, epigastric pain, and constipation have been associated with acetylcysteine. Call your medical practitioner about side effects.
Zolate - Clinical Pharmacology
Folates are best known for reducing the incidence of fetal neural tube defects (NTDs). NTDs are congenital malformations produced by failure of the neural tube to form and close properly during embryonic development. During the first four weeks of pregnancy—when many women do not even realize that they have conceived—adequate maternal folate intake is essential to reduce the risk of NTDs. As the postnatal period approaches there is increased demand again for folate regardless of lactation status.
Folate is involved in transformylation and methylation metabolism as well as (indirectly) succinylation metabolism (through the “methyl trap” hypothesis). Folate plays a central role in the formation of nucleic acid precursors, such as thymidylic acid and purine nucleotides, which are essential for nucleic acid synthesis and cell division. IOM/NAS (1998) noted that the evidence for a protective effect from folate supplements is much stronger than that for food folate. Other dietary ingredients are added to folate as cofactors, coenzymes and co-metabolites; in studies by Czeizel and Dudas (1992) and Berry et al. (1999), factors other than folate intakes may affect the magnitude of risk reduction or participate in a co-protective effect with folate.
Indications and Usage for Zolate
Zolate is indicated for the distinct nutritional requirements of patients in need of folate and Vitamin D supplementation as determined by a licensed medical practitioner.
Zolate should be administered under the supervision of a licensed medical practitioner.
Zolate Dosage and Administration
One (1) capsule daily or as directed by a licensed medical practitioner.
How is Zolate supplied
Zolate are clear gelatin capsules, and are supplied in bottles of 30 capsules.
| ZOLATE
folic acid, cholecalciferol capsule |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Axiom Pharma (079919011) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Carecam International, Inc. | 958024937 | manufacture(70033-113) , analysis(70033-113) , label(70033-113) , pack(70033-113) | |